Introduction
Traumatic diaphragmatic rupture and subsequent herniation of abdominal contents into the thoracic cavity is an uncommon clinical encounter typically associated with motor vehicle accidents or penetrating injuries (e.g., stab wounds, gunshot wounds, etc.). Blunt traumas are causative for approximately 75% of ruptures, while the remaining 25% are due to penetrating traumas.1 It is estimated that 0.8–1.6% of patients with blunt trauma develop diaphragmatic rupture,1 yet diaphragmatic injuries are often missed during the initial evaluation, with 12 – 66% of cases being overlooked.2, 3, 4 Right-sided diaphragmatic hernias are more likely to be missed.5, 6 To this end, it is essential to maintain a high degree of clinical suspicion for such injuries, particularly in patients suffering from thoracoabdominal or poly-traumatic injuries.
While the literature reports on cases of right traumatic diaphragmatic hernias from a surgical perspective, there is a scarcity of case reports on the condition from the anesthesiologist’s perspective. Through this report, we present a case of a male teen with a delayed presentation of a right diaphragmatic hernia, it's accompanying surgical approach, and anesthetic complications.
Case Report
A 17-year-old male (180 cm; 86 kg) with no comorbidities presented to the emergency department (ED) following a high-velocity, unrestricted, rear-ended motor vehicle crash (MVC). The patient initially complained of shoulder pain with subsequent development of agitation, tachycardia, hypotension, diaphoresis, and respiratory distress. Due to respiratory failure and a feared threat to his airway, the patient was intubated in the ED. Following an urgent chest X-ray and FAST scan, the patient was admitted to the ICU.
On admission, his vitals were as follows: temperature of 36.4°C, heart rate of 155 beats per minute (bpm), blood pressure of 82/60, and O2 saturation of 80–85%. His initial physical examination highlighted right upper-back and left lower-back abrasions, lungs clear to auscultations bilaterally, and Glasgow Coma Scores fluctuating between 7–13. No other remarkable findings were found.
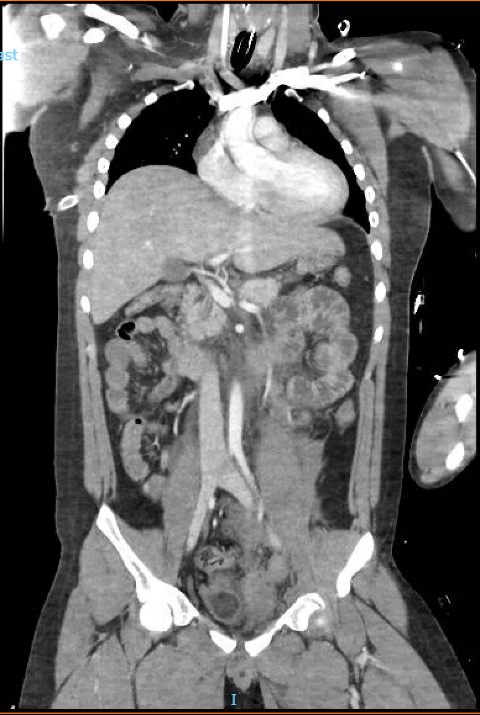
In the ED, a chest X-ray and a FAST scan were conducted, showcasing a small right hemothorax and no free fluid in the peritoneal or pericardial cavities, respectively. Following stabilization, a Computed Tomography (CT) of the Chest, Abdomen, and Pelvis was ordered (Figure 1), highlighting the following:
A small right pleural effusion with adjacent right lower lobe atelectasis, with a trace right pneumothorax.
Trace of left pleural effusion with adjacent left lower lobe atelectasis.
Non-enhancement of the entire left kidney on arterial and venous phases of imaging concerning for traumatic occlusion of the left renal artery.
Moderate-to-large left retroperitoneal hematoma extending inferiorly into the left hemipelvis along the course of the iliac vessels.
Small 1.9 × 0.9 cm contusion of the upper pole of the right kidney.
Displaced fractures of the left L1–L5 transverse processes and a mildly displaced fracture of the right L5 transverse process.
Minor fractures of the left inferior pubic ramus and upper left sacrum, with a widening of the left sacroiliac joint measuring 1.1 cm.
Small-to-moderate contained subcutaneous soft tissue hematoma overlying the lumbar spine.
The liver, gallbladder, bile ducts, heart, lymph nodes, and thyroid were unremarkable.
Following his admission, a chest tube was inserted to drain the hemothorax (~100 mL of blood drained), with serial chest X-rays showcasing gradual improvements of a right-sided layering pleural with no acute displaced rib fractures or a pneumothorax.
Over the next five days, the patient remained intubated and sedated in the ICU. A CT scan of the chest, abdomen, and pelvis was conducted two days after his admission, supporting the chest X-ray findings, redemonstrating the conclusions of the initial CT, and affirming that no liver, pancreas, gallbladder, or adrenal gland abnormalities were present.
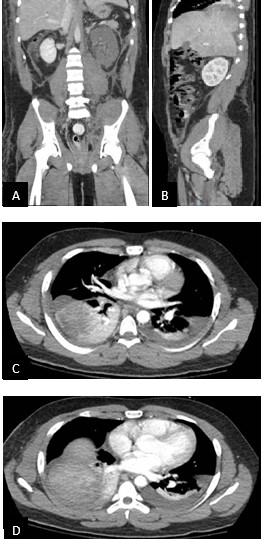
On day 6, the patient was extubated. However, he became increasingly hypoxic and tachypneic over the course of the next day. A CT of the chest and pelvis was conducted (Figure 2). There was no pulmonary embolism nor pneumothorax, but instead, the right lobe of the liver appeared to be bulging into the right hemithorax; a delayed right post-traumatic diaphragmatic hernia was diagnosed. This finding and the continued left kidney vascular abnormality were indications for an exploratory laparotomy.
Figure 2
(A and B) Coronal and CT of the chest and pelvis showcasing right herniation of the liver into the thoracic cavity; (C and D) Transverse CT of the right herniation of the liver into the thoracic cavity.
On pre-operative assessment, the patient was febrile (39.5°C), tachycardic (128 bpm), tachypneic (42 breaths/min), and moderately hypotensive (106/48). Physical and airway examinations were unremarkable, apart from decreased right breath sounds; he was classified as ASA Class IVE.
The patient was brought to the operating room to repair a complex liver herniation through the right hemidiaphragm. Under ultrasound guidance, a pre-induction arterial line was placed in the right radial artery. Following the placement of standard ASA monitors, the patient was pre-oxygenated to an end-tidal O2 of 80. Due to the level of patient discomfort, it was decided to perform induction of general anesthesia on the patient’s ICU bed in the operating room. The patient was placed into a slight reverse Trendelenburg position. Rapid sequence induction was performed using a combination of etomidate (10 mg) and propofol (60 mg) with succinylcholine. The first attempt at intubation by the in-room provider revealed a grade one view on laryngoscopy. However, after placement of the endotracheal tube, ventilation was noted to be difficult with the absence of end-tidal CO2. The staff anesthesiologist removed the endotracheal tube due to concern for esophageal intubation and made a second attempt at intubation utilizing a McGrath video laryngoscope. Video laryngoscopy revealed a grade one view, and the endotracheal tube was passed uneventfully. Following intubation, difficulty in ventilation and the absence of CO2 were noted again. Rapid desaturation began to occur.
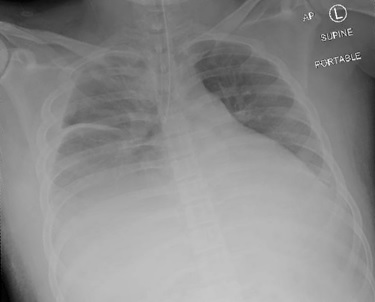
There was a concern for pulmonary compression from the enlarged liver herniation, creating difficulty in ventilation. The head of the bed was immediately raised to improve pulmonary compliance, and a significant improvement in ventilation with positive end-tidal CO2 was noted. Peak pressures at this time were noted to be elevated to approximately 35 cm H2O. Albuterol was administered through the endotracheal tube with slight improvement. The patient’s saturation improved to 100%. Bilateral breath sounds were noted on auscultation. Following the stabilization of the patient, we attempted to recline the head of the bed and noted significant difficulty in ventilation and elevation in peak airway pressures to ~50 cm H2O. An intraoperative chest X-ray was conducted to investigate the underlying cause. The X-ray showcased the endotracheal tube in the right bronchial mainstem (Figure 3). Subsequently, the patient was returned to a head-up position, and a fiberoptic bronchoscope was utilized to confirm the endotracheal tube placement 2 cm above the carina and patency of the airway. The patient’s oxygen saturation improved.
At this point, it was determined that the patient would not tolerate supine positioning for the duration of the procedure. An at-length discussion was held with the surgical team, and it was decided to repair the hernia in reverse Trendelenburg positioning. The patient was moved to the operating room table and placed in 28 degrees of reverse Trendelenberg positioning.
The surgery began with an upper midline laparotomy from the xiphoid process to the supraumbilical area. A Bookwalter retractor was placed to achieve better exposure of the abdominal cavity. Upon exploration of the right diaphragm, the liver was identified to be herniating through a large diaphragmatic defect. The defect was posterior and superior within the chest cavity, reaching close to the nipple level. Consequently, the initial incision was extended, with a transverse incision beginning at the middle of the midline incision. Further, the laparotomy incision was extended slightly inferior to the umbilicus. The aforementioned adjustments facilitated a full view of the liver and the defect. The patient remained hemodynamically stable throughout the hernia reduction and saturated well at 75% FIO2 with slight elevations in peak pressures in the 30–35 cm H2O range.
After the hernia reduction, it was noted that there was a significant drop in the peak airway pressures to ~20–25 cm H2O. At this point, the patient was able to tolerate supine positioning. Further, correction of the liver herniation allowed for a comprehensive visualization of the diaphragmatic defect: a linear injury measuring approximately 10 cm, beginning from the crux and extending laterally. Prior to the repair of the diaphragm, a right chest tube was placed. Upon aligning the two edges, adequate apposition was achieved without excess tension. A Babcock was used to hold the diaphragm’s edges together, and the repair was done using several Ethibond sutures in a figure-of-8 configuration.
Following the surgical repair of the hernia, a left nephrectomy, and closure of the abdomen, the decision was made to keep the patient intubated. He was transported to the ICU on monitors with ambu bag ventilation without complication.
Discussion
This report presents an unexpected case of delayed presentation of a right traumatic diaphragmatic hernia along with its surgical course. This report particularly discusses the unpredicted anesthetic emergency of ET displacement that occurred during its repair and the steps taken to ensure its timely resolution.
A diaphragmatic herniation is a documented complication of traumatic blunt abdominal trauma. In a study involving 1000 diaphragm injuries, 68.5% were left-sided, 24.2% were right-sided, and 1.5% were bilateral.1 Nonetheless, while rarer, right-sided diaphragmatic ruptures are a more difficult diagnostic challenge given their occult presentation, making them more likely to be missed while simultaneously being associated with higher mortality rates.5, 6 The liver’s presence may hide the initial defect on imaging, enabling the rupture to grow and lead to the eventual herniation of abdominal contents into the thorax.5 The imaging modality can influence early detection rates, with CT scans being preferred over chest X-rays. In a study involving 38 traumatic diaphragmatic hernias, chest X-rays were diagnostic in 30% of patients, while CT scans were diagnostic in 50% of patients.7 Similarly, in a case series involving 39 cases, chest X-ray was diagnostic in only 41% of patients, while CT scans of the chest and abdomen were 100% diagnostic.8
Yet, diaphragmatic hernias may be missed on CT scans as a hemothorax, intra-abdominal hematoma, lung collapse, lung contusions, or pneumothorax could obscure them.9 To this end, MRI has been proposed to enhance the detection of diaphragmatic hernias in hemodynamically stable patients due to its higher sensitivity and specificity, 10, 11 with CT scans remaining the modality of choice in emergency situations. Further, thoracoscopy is a valuable tool to ascertain the presence of diaphragmatic injuries, with accuracies ranging from 98–100%. In a retrospective review of 171 patients who underwent video-assisted thoracoscopic surgery following penetrating chest trauma, Freeman et al.12 outline five risk factors for DI:
Abnormal chest X-Ray at admission
Accompanying intra-abdominal trauma
High-velocity mechanism of injury
Entrance wound inferior to the nipple line
Right-sided entrance wounds.
Interestingly, intubation and positive pressure mechanical ventilation have been shown to reduce herniations through a diaphragmatic injury, facilitating hemodynamic improvements. Consequently, chest X-rays and CT scans of intubated patients are less likely to highlight DIs; this was exemplified in our case. Patients should undergo repeat imaging upon extubation if clinical suspicion of a diaphragmatic injury remains.9
From an anesthesiologist’s perspective, during a diaphragmatic hernia repair, approaches typically involve rapid sequence intubation to avoid mask ventilation and nasogastric tube insertion for gastric decompression. As described in our case, difficulties with ventilation may arise due to direct lung compression by the herniated visceral organs. The detrimental impact of herniation on a patient’s oxygenation and cardiovascular status cannot be understated; anesthesiologists must be wary of this potential complications. The forces applied by the organs may dislodge the ET tube into the right main bronchus as well. In such situations, the position of the ET tube should be confirmed with direct visualization through a fiber-optic bronchoscope before escalating management. Altering the patient’s position can also have a significant impact on facilitating adequate ventilation. Placing the patient in reverse-Trendelenburg or head-up position can reduce the presence of visceral organs in the thoracic cavity. The force the hernia has on the lung can be indirectly inferred from the peak airway pressures.
One-lung ventilation is another approach for repairing a right traumatic diaphragmatic, where it may be considered for improved surgical exposure and the theoretical prevention of mass effect exacerbations within the thoracic cavity. Re-expansion of the collapsed lung may lead to further mediastinal compression and cardiovascular deterioration. As such, Williams and Sandby-Thomas suggest one-lung ventilation using a double-lumen tube (DLT) or a single-lumen tracheal tube with bronchial blockers if a DLT is not available.13 However, one may need to anticipate several drawbacks of using DLTs, including being more time-consuming, higher risks of mispositioned tubes, failure of unilateral isolation, and increased risks of laryngeal injury.14
All in all, corrective surgery is the definitive treatment for traumatic diaphragmatic herniation. Given the direct impact of visceral organs on the lungs, temporary hypoxemia can be tolerated while optimizing pulmonary mechanics. The volatile nature of the operation must be discussed with the patients and their families, highlighting its potential risks and potential intra-operative complications.
Conclusion
We present a case of a delayed presentation of a right diaphragmatic traumatic hernia, the clinical course, and surgical and anesthetic management. It is a rare yet important complication of blunt abdominal trauma that requires a high degree of clinical suspicion to detect. Early diagnosis and repair are essential to prevent future complications such as strangulation and ischemia of intra-abdominal organs, lung collapse, and cardiovascular demise. The condition should be considered in all patients presenting with abdominal trauma and suspected should respiratory distress arises during the patient’s clinical course.
The recommended anesthetic approach is rapid sequence intubation with a cardiovascular-stable agent like etomidate and gastric decompression. Difficulties with intubation and ventilation should be anticipated due to the presence of the visceral organs within the thoracic cavity. This may manifest as hypoxemia and cardiovascular collapse. Altering the patient’s positioning (to reverse-Trendelenburg and head-up positioning) may be needed to facilitate adequate ventilation. Clear communication and flexibility among surgical and anesthetic teams are critical to ensure positive patient outcomes.